
15361669259

电 话:15361669259
手 机:15361669259
微 信:joeyhuang826
地 址:深圳市龙华区龙华大道2229号汇隆智尚B区338-1
joey-huang@foxmail.com
医疗器械设备产品分为三大类:1.诊断设备类,2.治疗设备类,3,辅助设备类。
贝科质量拥有全套的EMC电磁兼容、LVD安全检测设备,符合IEC/EN60601-1-2(电磁兼容)标准,IEC/EN60601-1(安规安全),GB9706.102 ,GB/T18268.1, GB/T18268.26。GB9706.1 的规范要求。可为医疗器械(医用电子设备)提供全套全项的检测服务,出具的报告可用于医疗设备注册或医疗设备备案.
EMC测试项目: 安规测试项目:
1、空间辐射Radiated 1、危险电压测试 12、异常工作测试
2、传导骚扰Conduction 2、防触电测试 13、扭力测试
3、谐波电流Harmonic 3、标签耐久性测试 14、冲击测试
4、电压闪烁Filcker 4、插头放电测试 15、跌落测试
5、静电ESD 5、保护接地测试 16、震动测试
6、电源快速脉冲群EFT/B 6、抗电强度测试
7、电源浪涌Surge 7、球压测试
8、工频磁场/磁场抗扰M/S 8、工作电压测试
9、交流电压跌落Voltage DIPS 9、电源线拉力测试
10、辐射抗干扰R/S 10、稳定性测试
11、电源传导抗扰度C/S 11、温升测试
生物相容性
生物相容性介绍:
生物相容性是指生物体组织对非活性材料产生反应的一种性能,一般是指材料与宿主之间的相容性,包括组织相容性和血液相容性医疗器械生物相容性评价较基本的内容之一是生物安全性,主要为生物学评价,因此,医疗器械生物相容性评价关注的重点是,医疗器械在和人体之间的相互作用下,是否对人体无毒性、无致敏性、无刺激性、无致癌性,对人体组织、血液、免疫等系统无不良反应。
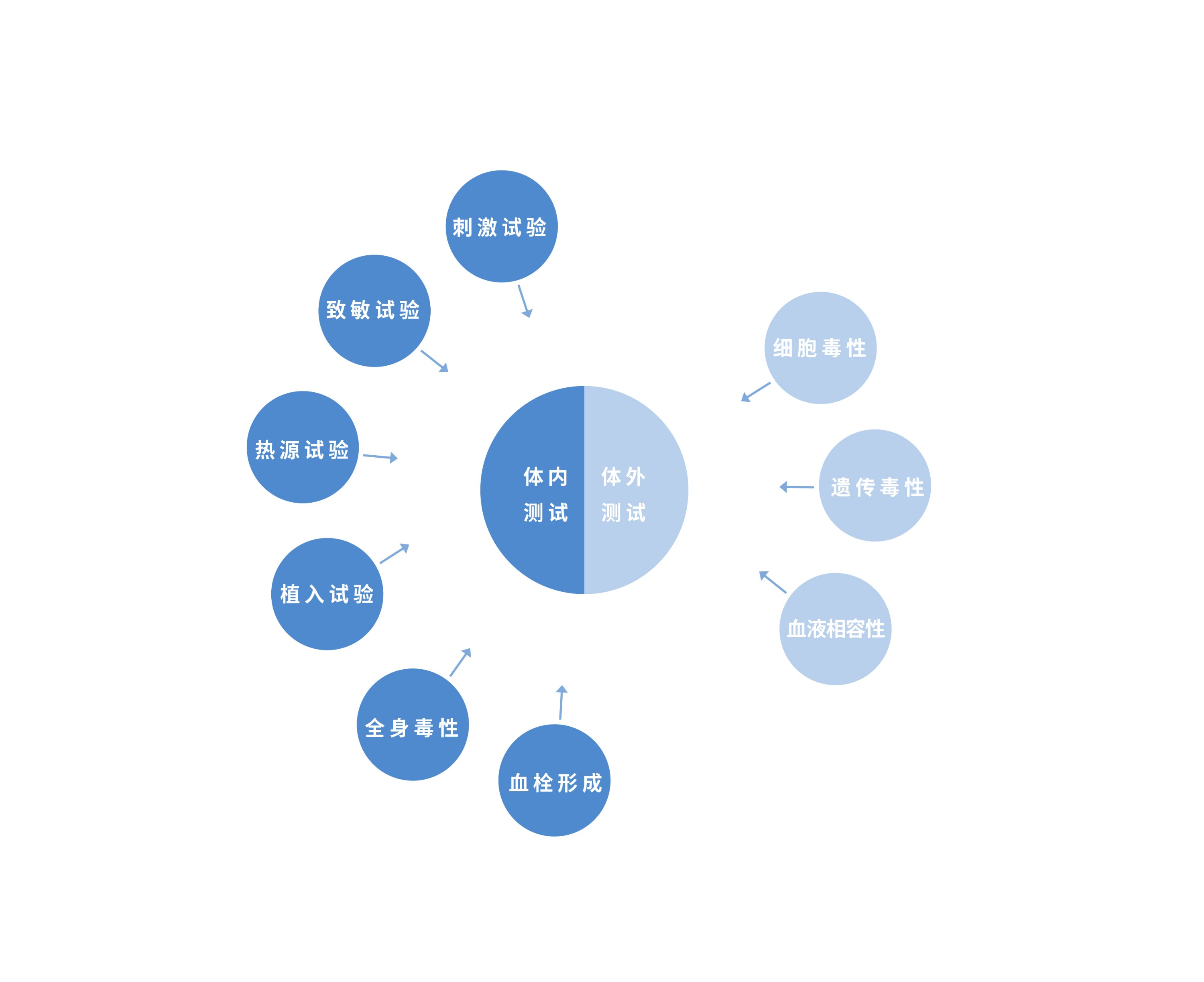
什么情况下要做生物相容性?
生物相容性测试项目比较多,主要有细胞毒性、致敏、刺激、全身毒性(急性毒性)、亚慢性毒性(亚急性毒性)、遗传毒性、植入、慢性毒性、致癌性、生殖和发育毒性和生物降解等。并不是所有的医疗器材产品都需要做全套的测试项目,行业只须要根据自身产品的使用特性,结合与身体触及的部位和时间长短,查寻符合自身产品的项目开展评判就可以了。事实上,对触及身体皮肤、粘膜和损伤表面等安全风险较低的医疗器材来说,只须要开展其中三个项目,分别是:体外细胞毒性实验、皮肤致敏性实验、皮肤刺激实验,也称生物学评判的基础3项。
当产品与身体长期触及或是触及部位风险较高的具体情况下,产品才需额外增加亚急/慢性毒性、遗传毒性、植入等实验。
项目及标准:
序号 | 项目 | 标准 |
1 | 体外细胞毒性试验 | ISO10993-5,GB16886.5 |
2 | 致敏试验 | ISO10993-10,GB16886.10 |
3 | 皮肤、皮内、口腔粘膜刺激试验 | ISO10993-10,GB16886.10 |
4 | 刺激试验 | ISO10993-23 |
5 | 急性全身毒性试验 | ISO10993-11,GB16886.11 |
6 | 亚急性全身毒性试验 | ISO10993-11,GB16886.11 |
7 | 亚慢性全身毒性试验 | ISO10993-11,GB16886.11 |
8 | 慢性全身毒性试验 | ISO10993-11,GB16886.11 |
9 | 热源试验 | ISO10993-11,GB16886.11 |
10 | 染色体畸变试验 | ISO10993-3,GB16886.3 |
11 | 微核试验 | ISO10993-3,GB16886.3 |
12 | 基因突变试验 | ISO10993-3,GB16886.3 |
13 | Ames试验 | ISO10993-3,GB16886.3 |
14 | 血栓形成试验 | ISO10993-4,GB16886.4 |
15 | 凝血试验 | ISO10993-4,GB16886.4 |
16 | 血小板粘附试验 | ISO10993-4,GB16886.4 |
17 | 补体激活试验 | ISO10993-4,GB16886.4 |
18 | 溶血试验 | ISO10993-4,GB16886.4 |
19 | 肌肉植入试验 | ISO10993-6,GB16886.6 |
20 | 皮下植入试验 | ISO10993-6,GB16886.6 |
21 | 骨植入试验 | ISO10993-6,GB16886.6 |
22 | 材料特征分析 | ISO10993-18 |
… | … | … |
名称/Title | 国际标准/international standards | 国内标准/National standards |
测量、控制和试验室用电气设备的安全要求 Electrical Equipment For Measurement, | IEC/EN 61010-1 | GB4793.1 |
测量、控制和试验室用电气设备的安全要求 Safety requirements for electrical equipment for measurement, control andlaboratory use –Part 2-101: Particular requirements for in vitro diagnostic (IVD) medicalequipm |
IEC/EN 61010-2-101
| YY 0648 |
测量、控制和实验室用电气设备的安全要求:实验室用分析和其他目的自动和半自动设备的特殊要求 Safety requirements for electrical equipment for measurement, control and laboratory use – Part 081: Particular requirements for automatic and semiautomatic laboratory equipment for analysis and other purposes |
IEC/EN 61010-2-081
| GB4793.9 GB/T42125.14 |
测量、控制和实验室用的电设备 电磁兼容性要求 第1部分:通用要求 Electrical equipment for measurement, control and laboratory use - EMC requirements - Part 1: General requirements |
IEC/EN 61326-1
| GB/T 18268.1 |
测量、控制和实验室用电设备.电磁兼容性(EMC)的要求.特殊要求.实验室诊断 Electrical equipment for measurement, control and laboratory use - EMC requirements - Part 2-6: Particular requirements - In vitro diagnostic (IVD) medical equipment |
IEC/EN 61326-2-6
| GB/T18268.26 |
名称/Title | 国际标准/international standards | 国内标准/National standards |
医用电气设备 第2部分 心电图机安全专用要求 Medical safety and electrical essential equipment performance - Part 2-25: of electrocardiographs Particular requirements | IEC/EN 60601-2-25 | GB 10793 |
无创血压计第1部分:通用要求 Non-invasive sphygmomanometers - Part 1: Requirements and test methods for non-automated measurement type | EN ISO /ISO 81060-1 | |
无创血压计.第3部分:电-机血压测量系统的补充要求 Non-invasive sphygmomanometers - Part 3: Supplementary requirements for electro-mechanical blood pressure measuring systems |
EN 1060-3
| |
医用电气设备 第2-27部分:心电监护安全及基本性能专用要求 Medical electrical equipment - Part 2-27: Particular requirements for the basic safety and essential performance of electrocardiographic monitoring equipment |
IEC/EN 60601-2-27
| GB 9706.25 |
医用电气设备 第2-26部分:脑电图机安全专用要求 Medical electrical equipment-Part 2-26: Particular requirements for the safety of electroencephalographs | IEC/EN 60601-2-26:2012 | GB 9706.26 |
医用电气设备 第2-56部分:临床体温计的基本安全和性能的专用要求 Medical safety and electrical essential equipment-Part performance2-56: of clinical Particular thermometers requirements for basic body temperature measurement | ISO/ EN ISO 80601-2-56 | YY 9706.256 |
血压计 Non-invasive automated sphygmomanometers | ANSI/AAMI SP10 | YY 0670 |
医用电气设备--第2-57部分:治疗、诊断、监测和美容/美学使用的非激光光源设备的基本安全和基本性能专用要求 Medical electrical equipment - Part 2-57: Particular requirements for the basic safety and essential performance of non-laser light source equipment intended for therapeutic,diagnostic,monitoring and cosmetic/aesthetic use |
IEC/EN 60601-2-57
| YY 9706.257 |
医用电气设备 第2-2部分:高频手术设备安全专用要求 Medical Basic Safety Electrical And Equipment Essential -Performance Part 2-2: Particular Of High Requirements Frequency For Surgical Equipment And High Frequency Surgical Accessories | IEC/EN/EN IEC 60601-2-2 | GB 9706.4 |
医用电气设备第1部分:安全通用要求 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance | ANSI/AAMI ES60601-1 | GB9706.1 |
医用电气设备第2-10部分:神经和肌肉刺激器安全专用要求 Medical electrical equipment - Part 2-10 : particular requirements for the safety of nerve and muscle stimulators |
IEC/EN 60601-2-10
| YY 0607 |
医用电气设备 第2-30部分:自动循环无创血压监护设备的安全和基本性能专用要求 Medical electrical equipment - Part 2-30: Particular requirements for basic safety and essential performance of automated non-invasive sphygmomanometers |
| YY 0667 |
医用电气设备 第2-49部分:多参数患者监护设备安全专用要求 Medical electrical equipment-Part 2-49:Particular requiremens for the safety of multifunction patient monitoring equipment | IEC/EN 80601-2-49 | YY 0668 |
医用电气设备.医用脉搏血氧仪设备基本安全和主要性能专用要求 Medical electrical equipment — Part 2-61: Particular requirements for basic safetyand essential performance of pulseoximeter equipment |
ISO / EN ISO 80601-2-61
| YY 0784 |
医用电气设备 第2-38部分:医院电动床安全专用要求 Medical electrical equipment - Part 2: Particular requirements for the safety of electrically operated hospital beds |
IEC/EN 60601-2-52
| YY 0571 |
心电诊断设备 Diagnostic electrocardiographic devices | ANSI/AAMI EC11 | YY 1139 |
医用电气设备 第1-11部分:安全及基本性能的通用要求—并列标准:家庭医疗保健环境下使用的医用电气设备和系统 Part 1-11: General requirements for basic safety and essential performance –Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment |
IEC/EN 60601-1-11
| YY 9706.111 |
医用体温计.最大装置小型电体温计(非预测型和预测型)的性能 Performance of compact electrical thermometers (non-predictive and predictive) with maximum device | EN 12470-3 | |
临床体温计.连续测量用电子体温计的性能 Performance of electrical thermometers | EN 12470-4 | YY 0785 |
红外体温计 infrared ear thermometers (with maximum device) | EN 12470-5 | GB/T 21417.1 |
医用电气设备 第2部分:动态心电图系统安全和基本性能专用要求 Part 2-47: Particular requirements for the basic safety and essential performance of ambulatory electrocardiographic systems | IEC/EN 60601-2-47 | YY 0885 |
医用电气设备 第2部分:诊断和治疗激光设备安全专用要求 Part 2-22: Particular requirements for basic safety and essential performance of surgical,cosmetic,therapeutic and diagnostic laser equipment |
IEC/EN 60601-2-22
| GB 9706.20 |
医用电气设备.第2部分:手术无影灯和诊断用照明灯安全专用要求 Part 2-41: Particular requirements for the basic safety and essential performance of surgical luminaires and luminaires for diagnosis | IEC/EN 60601-2-41 | YY 0627 |
医用电气设备 第2部分:手术台安全专用要求 Part 2-46: Particular requirements for the basic safety and essential performance of operating tables |
IEC/EN 60601-2-46
| YY 0570 |
心电监护仪电缆和导联线 ECG trunk cables and patient leadwires | AAMI ANSI EC53 | YY 0828 |
医用电气设备 第2-18部分:内窥镜设备安全专用要求 Part performance 2-18: Particular of endoscopic requirements equipment |
IEC/EN 60601-2-18
| GB9706.19 |
医用电气设备 第1-2 部分:安全通用要求 并列标准:电磁兼容 要求和试验 Medical electrical equipment-Part 1-2:General requirements for safetyCollateral standard :Electromagnetic compatibility-Requirements and tests |
IEC/EN 60601-1-2
| YY 0505 YY9706.102 |
医用电气设备 - 第1-8 部分:基本安全和基本性能的通用要求 - 并列标准: 通用要求,医疗电气设备和医疗电气系中统报警系统的测试和指南 Medical electrical equipment- Part 1-8: General requirements for safety Collateral standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems |
IEC/EN 60601-1-8
| YY0709 |
激光产品的安全 第1 部分:设备分类和要求 Safety of laser products —Part 1: Equipment classification and requirements | IEC/EN 60825-1 AS/NZS 2211.1 | GB 7247.1 |
灯和灯系统的光生物安全性 Photobiological safety of lamps and lamp systems | IEC /EN62471 | GB/T 20145 |
呼吸治疗设备,第1部分:雾化系统及其组成部分RESPIRATORY THERAPY EQUIPMENT –Part 1: Nebulizing systems and their components | EN13544-1 | YY 0109 |
医用电气设备--第2-24部分:输液泵及控制器的基本安全和基本性能专用要求 Medical electrical equipment –Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers |
IEC/EN 60601-2-24
| GB 9706.27 |
医用电气设备--第2-34部分:有创血压监护设备的基本安全和基本性能专用要求 Medical electrical equipment - Part 2-34: Particular requirementsfor the basic safety and essential performance of invasive bloodpressure monitoring equipment |
IEC/EN 60601-2-34
| YY0783 |
医用电气设备 2-60部分:牙科设备的基本安全和基本性能 Medical electrical equipment –Part 2-60: Particular requirements for the basic safety and essential performance of dental equipment |
IEC/EN 80601-2-60
| GB 9706.260 |
医用设备 - 第一部分:医用设备可用性工程的应用 Medical devices IEC devices 62366-1: - Part 2015 1: IEC Application 62366: 2007+A1:2014 of usability engineering |
IEC/EN 62366-1
| YY/T 1474 |
医用电气设备 - 第1-6部分:基本安全和基本性能的通用要求 - 并列标准:可用性 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral s tandard: Usability |
IEC/EN 60601-1-6
| YY/T 9706.106 |
医用电气设备 第2部分:婴儿培养箱安全专用要求 Medical electrical equipment -Part 2:Particular requirements for safety of baby incubators |
IEC/EN 60601-2-19
| GB 9706.219 |
医疗电气设备-第1-9部分:基本安全和重要性能的一般要求.附属标准:环境意识设计的要求 Medical electrical equipment Part 1-9: General requirements for basic safety and essential performance – Collateral Standard: Requirements for environmentally conscious design |
IEC/EN 60601-1-9
| |
医用电子体温计 Clinical electronic thermometer | GB/T21416 | |
医疗器械软件 软件生存周期过程 Medical device software — Software life cycle processes |
IEC62304
| YY/T 0664 |
医用吸引设备 第1部分: 电动吸引设备 安全要求 Medical suction equipment Part 1: Electrically powered suction equipment — Safety requirements |
ISO10079-1
| YY/T 0636.1 |
医用电气设备 第2-37部分:超声医疗诊断监视设备基本安全和基本性能的特殊要求 Medical electrical equipment —Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment | IEC/EN 60601-2-37 | GB 9706.9 |
Standard means for the reporting of the acoustic output of medical diagnostic ultrasonic equipment 医用超声诊断设备声输出公布要求
| IEC 61157 | GB/T16846 |
医疗诊断超声波设备的声输出报告用标准方法 Ultrasonics dental descaler systems; measurement and declaration of the output characteristics | IEC 61205 | YY/T 0751 |
超声多普勒胎儿心率仪 ultrasonic Doppler fetal heartbeat detector | YY 0448 | |
超声多普勒胎儿监护仪 ultrasonic Doppler fetal monitor | YY/T0449 | |
超声理疗设备 Medical electrical equipment —Part 2-5: Particular requirements for the basic safety and essential performance of ultrasonic physiotherapy equipment | IEC/EN 60601-2-5 | YY/T1090 |
超声 水听器 第1部分:40MHz 以下医用超声场的测量和特征描绘 ultrasonic-Hydrophone Part 1: Measurement and characterization of medical ultrasonic fields to 40MHZ | IEC62127-1 | YY/T 0865.1 |
医用内窥镜 | IEC/EN 60601-2-18 | YY 0068.1, YY 0068.2, YY/T 0068.3 ,YY 0068.4 |
医用胶囊式内窥镜 | YY 1298 | |
医用内窥镜照明用光缆 | YY/T 0763 | |
医用电子内窥镜 | YY 1587 | |
医用内窥镜 内窥镜功能供给装置 摄像系统 | YY/T1603 | |
环境试验要求及试验方法 | GB/T14710 | |
产品加速老化试验方法 | IEC62506 | GB/T34986 |
Copyright © 2024 深圳市贝科质量技术服务有限公司 版权所有 粤ICP备2024268816号 网站地图